
AnalyticsBionicsElectrodics
MicrofluidicsReverse
ElectroDialysisInterDigitated ArrayIontronicsMulti-Phase ElectrochemistrySynaptic InterfaceElectron transfer kineticsFTEISProbe ElectrochemistryPhage ElectrochemistryPhotoelectrocatalysisSiO₂Organic Electrochemistry Microfluidics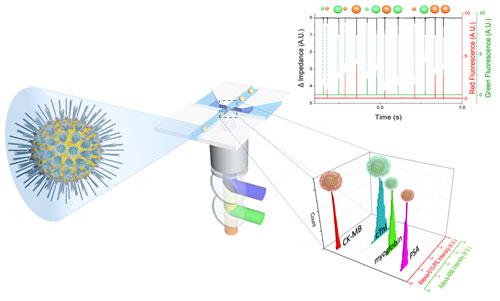
Modern analytical chemistry is facing new challenges. One of the urgent targets to cope with the challenges is multiplex analysis requiring as small a sample volume as possible. In this situation, we paid attention to suspension arrays that employ microbeads. Bead-based suspension arrays for common use require enhanced sensitivity and effective prevention of non-specific adsorption, as well as miniaturization of the detection device. We have implemented virus-tethered gold microspheres for multiplex immunoassay applications, employing a DC impedance-based flow cytometer as a detection element. The advantages of virus-tethered gold microspheres, including excellent prevention of non-specific adsorption, are extended to signal enhancement arising from the large quantity of antibody loading on each virion, and to flexible movement of filamentous virus. Individual virus-tethered beads generate their own DC impedance and fluorescence signals, which are simultaneously detected by a chip-based microfluidic flow cytometer. This system successfully realized multiplex immunoassays involving four biomarkers: cardiac troponin I (cTnI), prostate specific antigen (PSA), creatine kinase MB (CK-MB), and myoglobin in undiluted human sera, elevating sensitivity by up to 5.7-fold compared to the beads without virus. Constructive integration between filamentous virus-tethered Au-layered microspheres and use of a microfluidic cytometer suggests a promising strategy for competitive multiplex immunoassay development based on suspension arrays. RED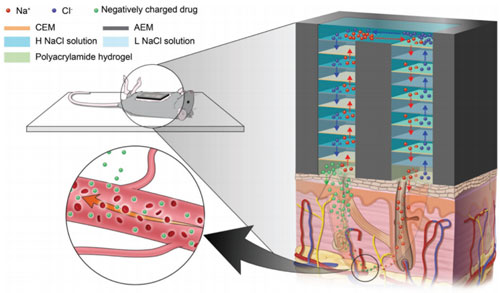 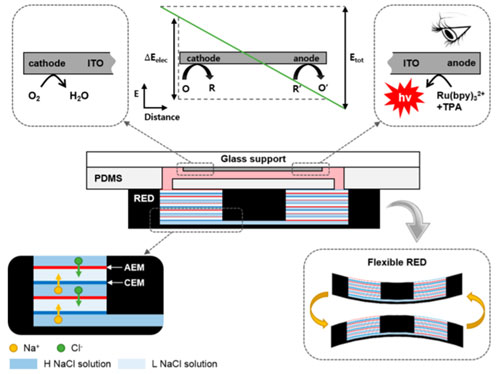 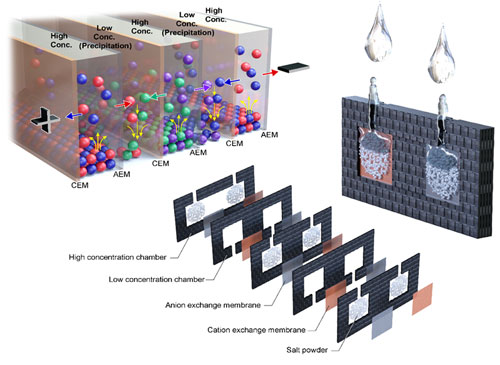
Reverse electrodialysis (RED) is a energy generating system from the salinity gradient of sea water and river water. We focus on the fact that RED is an ideal power source surrogate to couple with various ionic devices without the need of any metal electrodes. Thus, we developed a miniaturized RED to be used as a power source for a drug delivery system and that of sensors, Conceptual improvement was also made via a precipitation-based approach to enhance its practicality. Now, we are attempting to expand its applications to a wide range of ion-based platforms. Analytical platform3D Interdigitated array Electrodes(3D IDA)Sensitivity is significant criteria for biosensors. We devised three-dimensional interdigitated array (3D IDA) platform for amplifying the current signal to enhance the sensitivity of chip-based electrochemical immunosensors. The 3D IDA consists of one pair of IDA which are positioned on the bottom and the ceiling facing each other in a microfluidic channel. Comparing with the case of feedback mode and non-feedback mode, the current signal of feedback mode was ca. 100 times higher than that of non-feedback mode. We also demonstrated the 3D IDA chip that operate as electrochemical immunosensors without reference and counter electrodes, which are essential components to control appllied potential but prevent users from being free to detect targets on-site. Electron transfer mediator is immobilized on the one pair of working electrodes and the electrochemical potential of the electrodes are defined by the formal potential of the mediators in 2-electrode (2E) system. Therefore, the 3D IDA in 2E system constitutes a simple platform for sensitive electrochemical detection and sheds light on miniaturized multiplex immunoassay field. 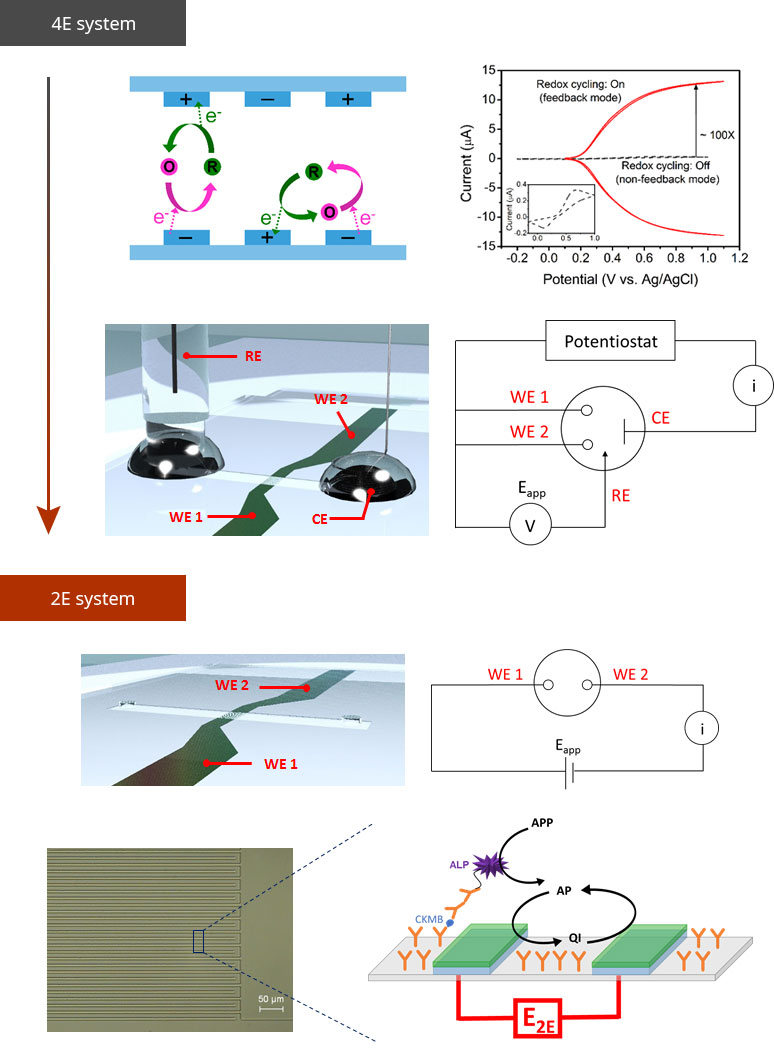 Iontronics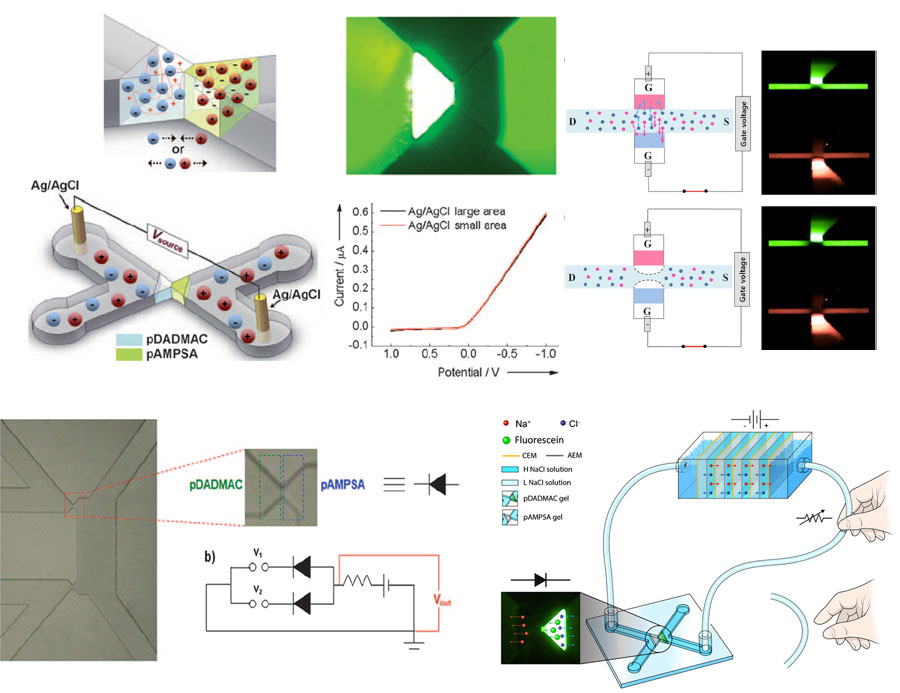
Iontronics, as the name reminds us of its meaning, mainly deals with electrical signal processing based on ions as signal carriers under aqueous, physiological conditions, and thereby aims to realize a bio-inspired information processing units. For the purpose of a sophisticated control of ions, positively- or negatively charged polyelectrolyte gels are used as a key component in the microfluidic chip. By optimizing the electrochemical circuit design and materials, we demonstrated that systematic combinations of such hydrogels produced the first aqueous microfluidic ion diode, digital logic gates and a polyelectrolytic junction field effect transistor (pJFET). Recently, we have constructed a full ionic circuitry with combination of reverse electrodialysis (RED) as an ionic power source, which is completely composed of non-electronic materials. We envision futuristic ionic devices capable of neurological signal processing functions on biocompatible substrates such as starch paper. Virtual ElectrodeVirtual Electrode
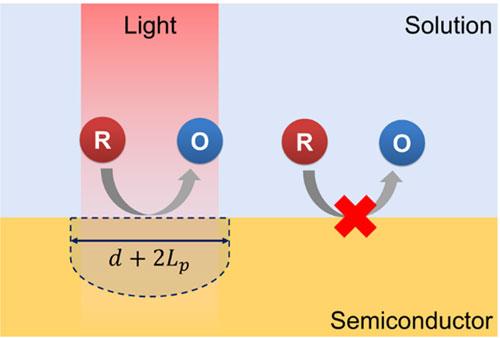
When the semiconductor absorbs photons of high enough energy, electron−hole pairs are created. Among them, the minority carriers migrate to the semiconductor surface and react with the redox species in the solution. Most photoelectrochemistry research has been focused on the production of environmentally friendly fuels taking advantage of the power of semiconductors, to convert solar energy into chemical energy. However, the characteristics of the semiconductor we are interested in is the 'transient' nature of photo-generated excitons. Excitons exclusively exist when and where the light illuminates. Therefore, the photoelectrochemical reactions are also spatiotemporally confined to the illumination. Thus, it is possible to induce a “virtual” electrode on the semiconductor substrate wherever and whenever we want without complicated electrical wiring process or the electrode-positioning procedure. So far, we have showed some applications based on virtual electrode including large scale, maskless metal patterning with amorphous silicon and microscale biomolecule detection with hematite photoanode. Maskless PatterningBiomolecule detection닫기Bio Fuel Cell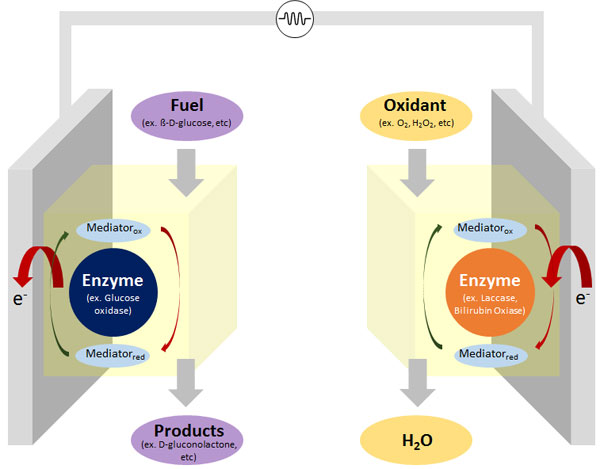
Enzymatic biofuel cell is fuel cell that utilizes enzymes as biocatalyst to oxidize its fuel such as glucose and reduce its oxidant to water to harvest energy. At anode, the electrons are separated from the fuel molecules by enzyme and transfer to the anode electrode. At cathode, those electrons transfer to the enzyme and react with the oxidant.
Multi-Phase Electrochemistry
The Br-/Br2 redox couple in aqueous solution are being studied for redox flow batterys (figure 1). N-methyl-N-ethyl pyrrolidinium bromide (MEPBr) is used in Bromide redox system as bromine-complexing agent, forms an immiscible emulsion to overcome the self-discharge, corrosion and toxicity of Bromine. In recent studies, It is revealed that Bromide is oxidized in not only aqueous solution but also MEPBr2n+1, and there is a large amount of Bromide in the MEPBr2n+1 phase. In addition, We observed that when a single droplet of MEPBr2n+1 collides with electrode, bromide oxidizes immediately and spike-shaped current is occurred using in situ optical and electrochemical tools (figure 2). In the future, we will going to study electrochemistry of single droplet entity and compare the difference from the emulsion which is ensemble of the droplets. Synaptic Interface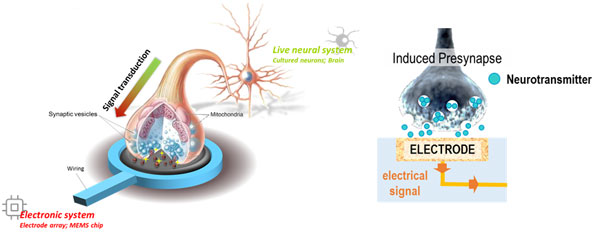
Neurons communicate with each other using chemical signals through synapses. We envision a future where communicating with neurons is much easier. With this hope, we propose Janus synapses as a novel neural interfacing method. Just as neurons do, electronic devices and single neurons can establish connections for chemicalcommunication through synapses. This method involves pairing single neurons with counterpart electrodes. These electrodes induce the neurons to form synapses, differentiating intoeither presynaptic or postsynaptic terminals. 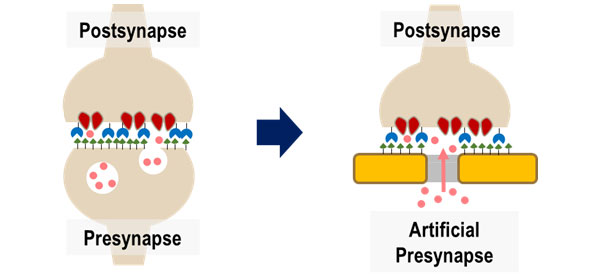
One strategy we adopt is establishing synaptic connections between neurons and electrodes mediated by synaptic cell adhesion. Synaptic cell adhesion molecules are known to be involved in synapse formation. We have genetically engineered several of these molecules, including neuroligin (NLG), slitrk, and neurexin (NRX), to bind to electrode surfaces. Over the past several years, our lab has evaluated the synaptogenic abilities of these recombinant proteins. Interestingly, the recombinant postsynaptic proteins (neuroligin1, neuroligin2, and slitrk3) seems to induce different types of presynaptic terminals even when immobilized on artificial substrates such as electrodes and microspheres. These synaptic types include glutamatergic (excitatory), GABAergic (inhibitory), and dopaminergic. In this system, a presynapse forms spontaneously on a functionalized electrode, and exocytotic chemicals are directly released to the electrode. This allows chemical signals to be converted into electrical signals. With synapse type specificity, we monitor neuronal signals, particularly neurotransmitters, in Janus synapses using electrochemical methods. The Janus synapse is expected to be very robust against neuronal migration and glial penetration because the induced synapse is firmly bound to the electrode surface through protein-protein interaction. 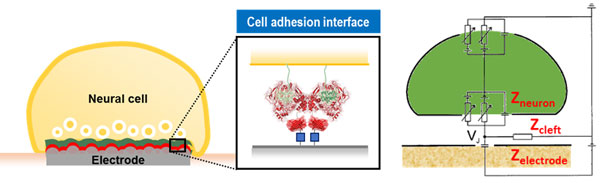
Iontronic (ionic) or electrical stimulation methods are commonly used in neuroscience research for studying neuronal activity and modulating neural function. Recently, our laboratory has discovered an innovative application of inverted ion current rectification phenomenon in stimulating neurons through devices, presenting promising advantages. This approach enables quantitative neurotransmitter delivery with high temporal resolution, negligible leakage, and no mechanical stimulation by using a polyelectrolyte-filled micropipette. Electron transfer kinetics
Investigating the electron-transfer kinetics of rapid outer-sphere reactions using voltammetric techniques presents a significant experimental hurdle. This is primarily due to the fact that voltammetric measurements only detect heterogeneous kinetics effectively when the rates of electron transfer and mass transport to the working electrode reach a similar level, a condition that is hard to meet for fast reactions with standard electrochemical equipment. We have addressed this challenge by fabricating two parallel electrodes facing each other with narrow gaps down to 10 nm, allowing us to observe the redox behavior of electrochemically active molecules confined between them. This platform enables precise verification and control of the electrode geometry, ensures stable operation, and provides opportunities for analyzing electron transfer kinetics within an extended potential window. FTEIS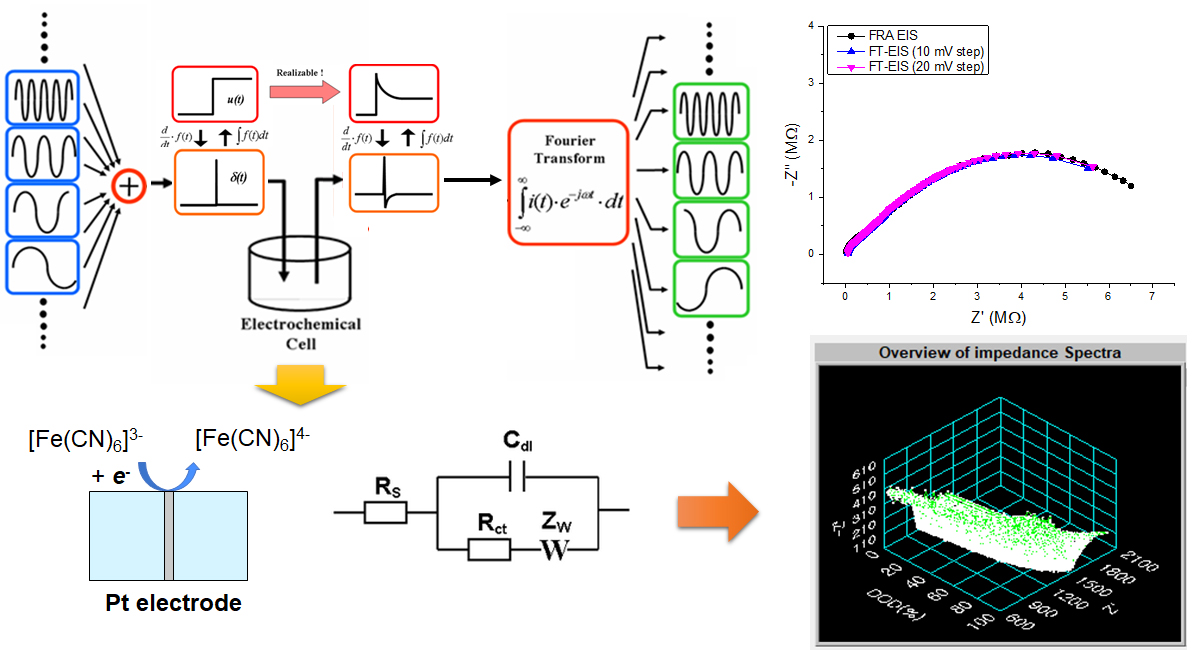
Fourier transform electrochemical impedance spectroscopy (FT-EIS) can be utilized as a powerful tool to a number of applications where the change of components in equivalent circuits should be examined in real-time manner. This technique allows for a very short EIS measurement time by applying a small (5~15 mV) voltage step signal to the system, and thereby calculating impedance values for the frequency range of interest through mathematical signal processing. Among its prospective applications are the fundamental study of electrochemical system, transient electrode process such as etching, electroplating, corrosion, and usages as a novel analytical platform. Probe Electrochemistry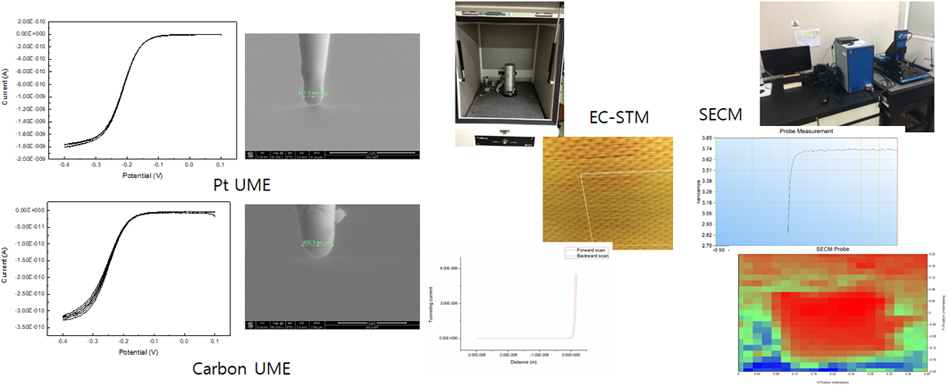 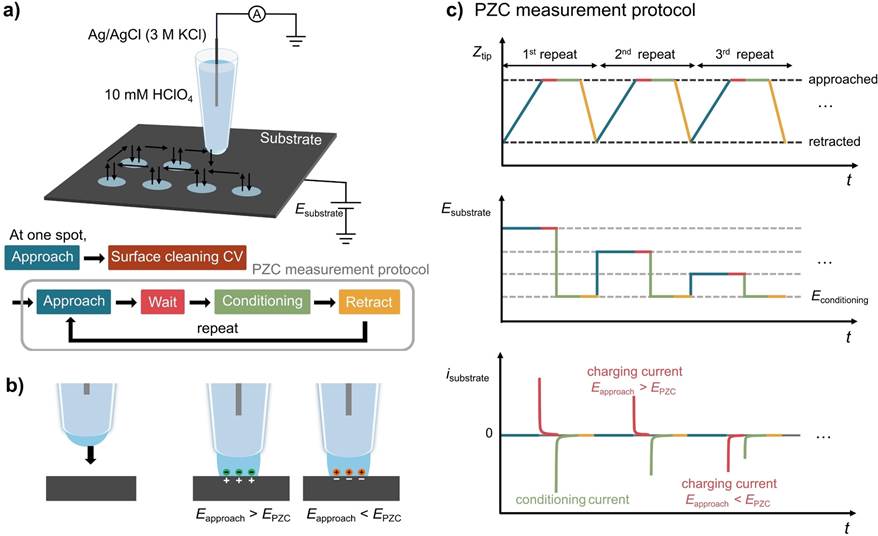
We have also installed sophisticated Scanning Ion Conductance Microscopy (SICM) and Scanning Electrochemical Cell Microscopy (SECCM) instruments based on LabVIEW, which allow us to conduct electrochemical experiments in various ways different from conventional SICM/SECCM. Recently, we have introduced polyelectrolyte gel-filled pipettes to SICM/SECCM, analyzing electrochemical interfaces such as measuring surface charge density or local potential of zero charge. Phage ElectrochemistryApplication of Filamentous Bacteriophage& Gold microshell
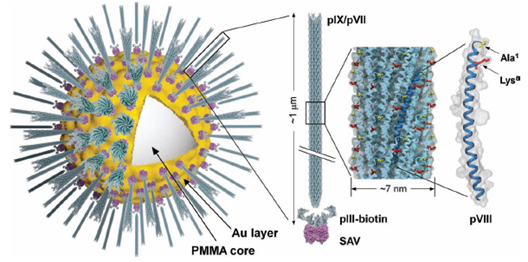 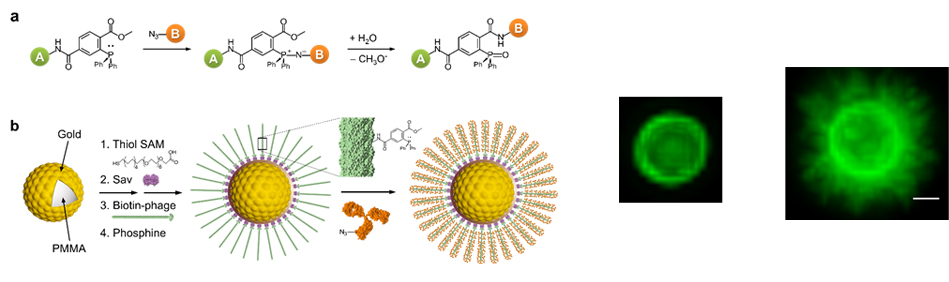 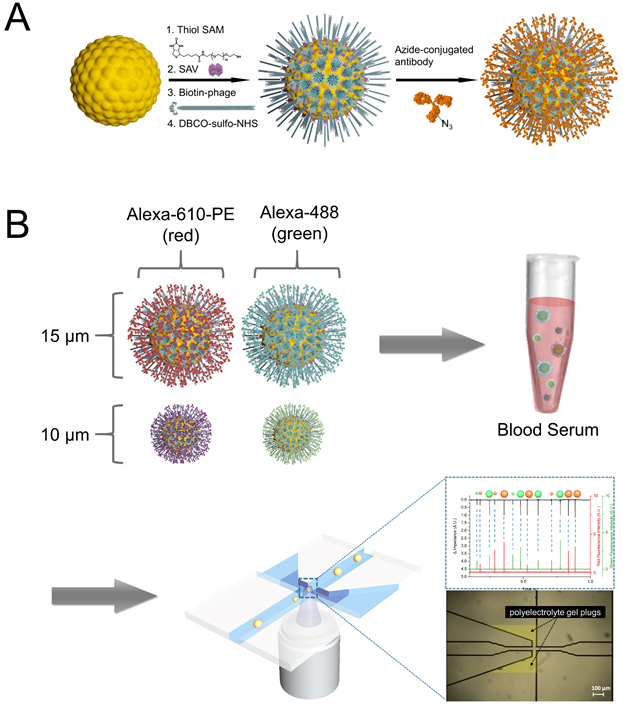 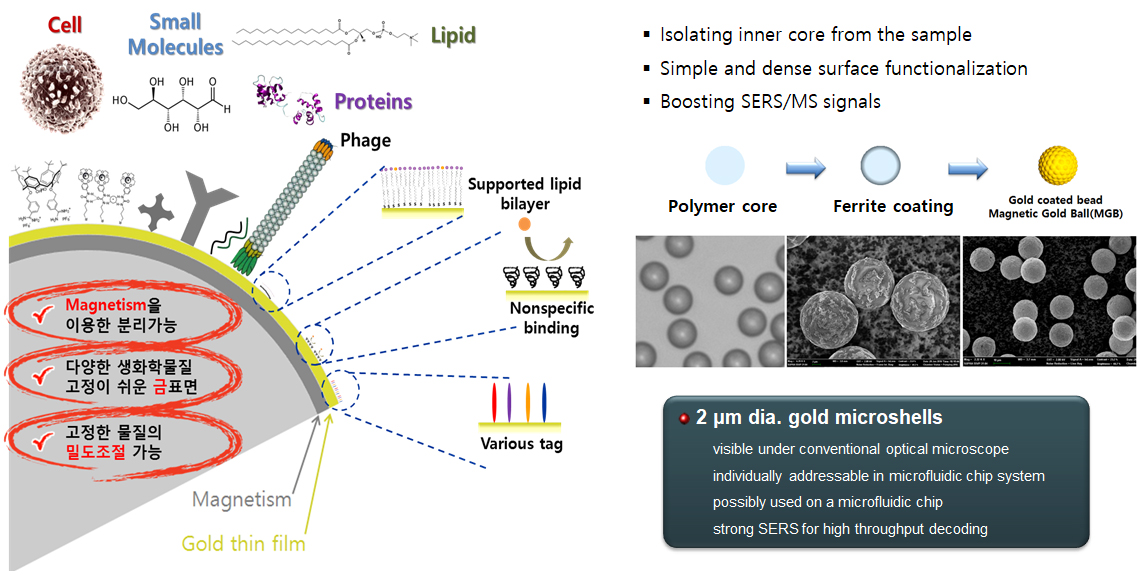 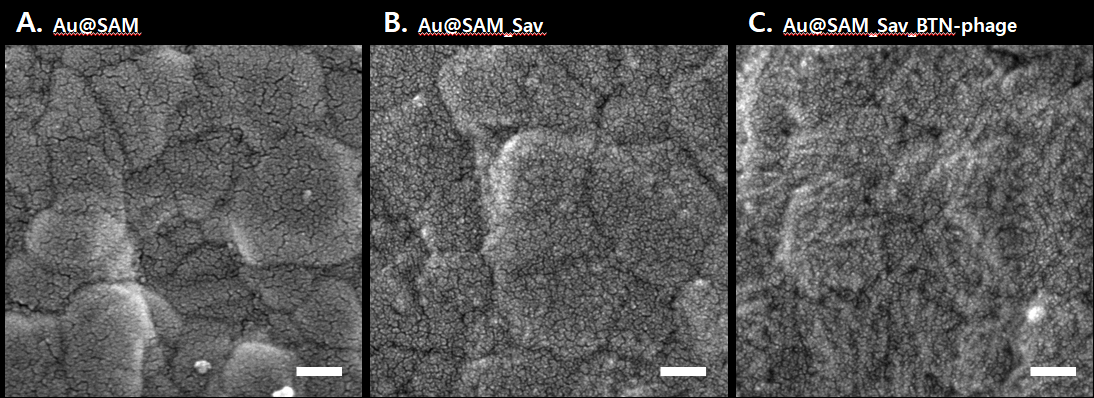 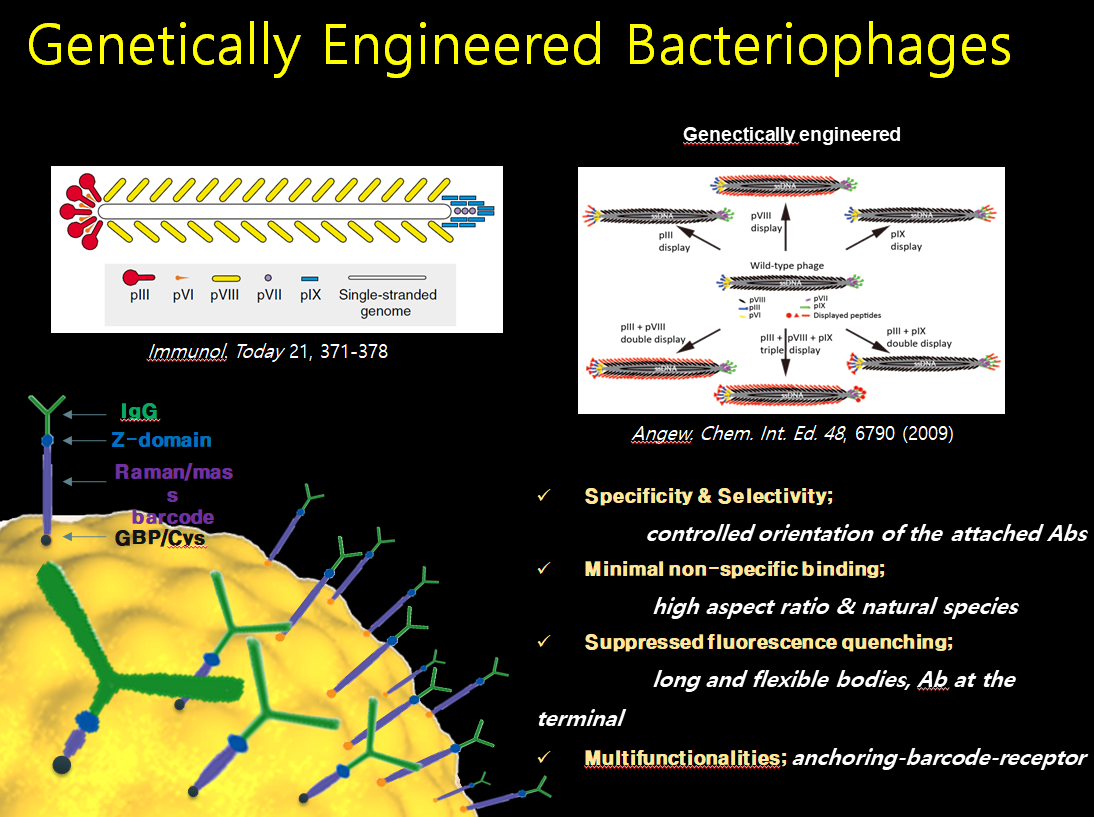 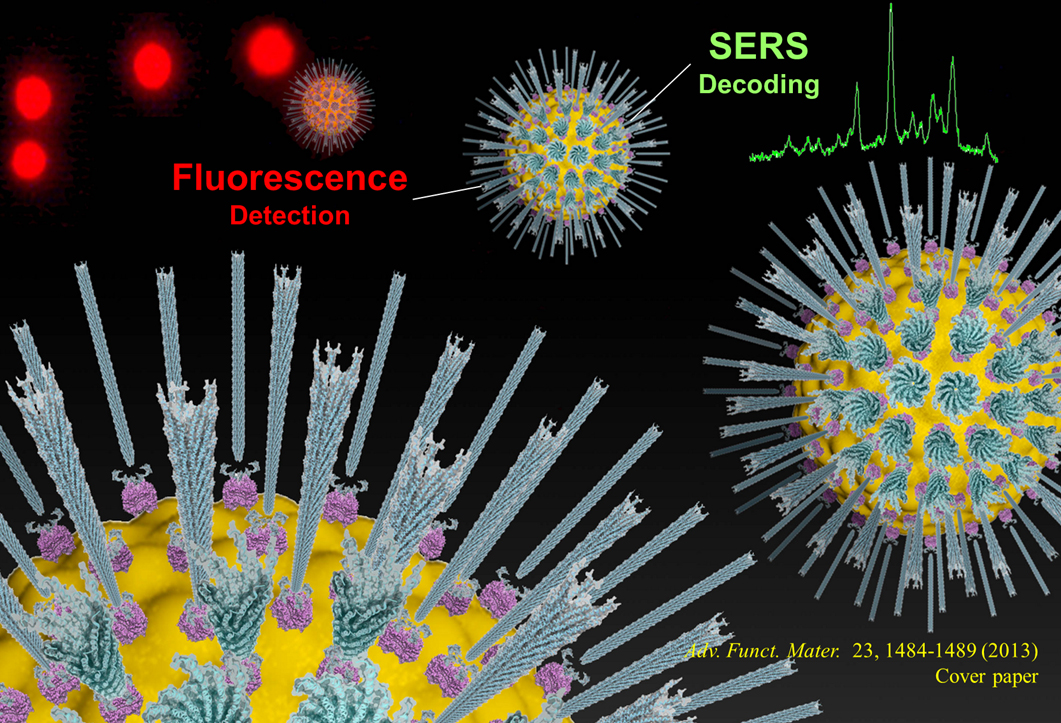 Filamentous bacteriophage as a template for OER catalyst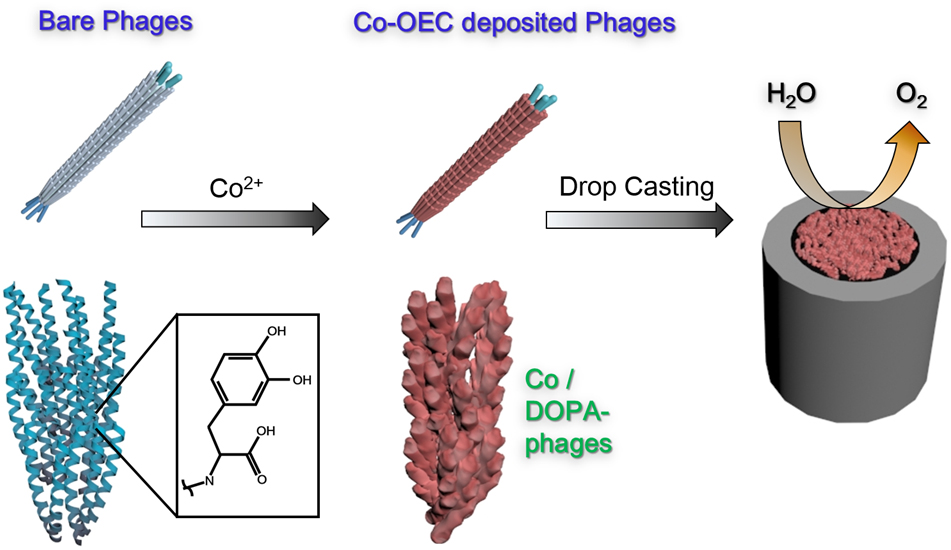 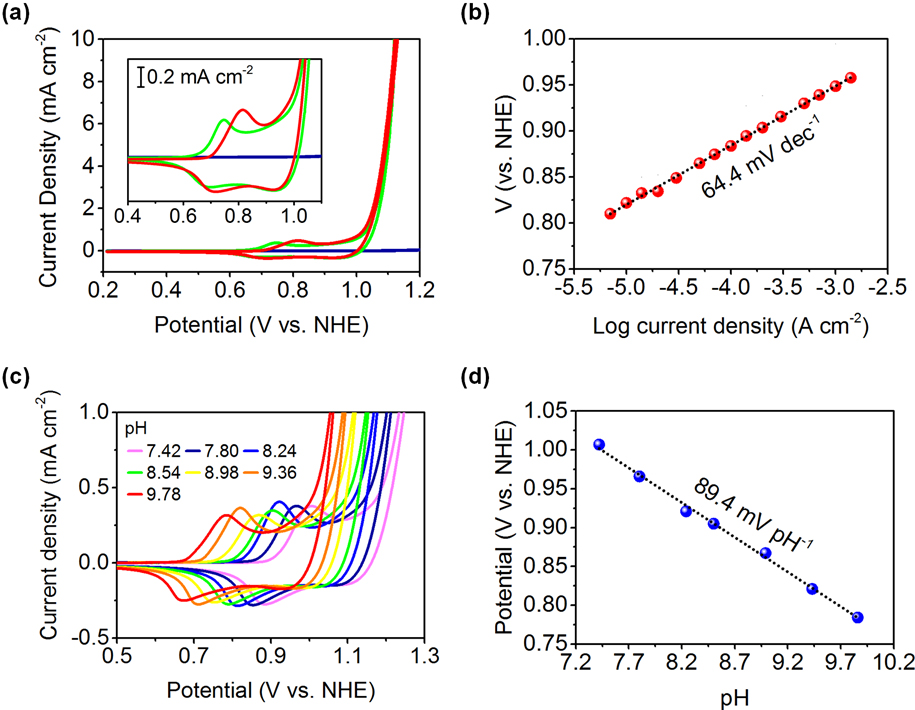 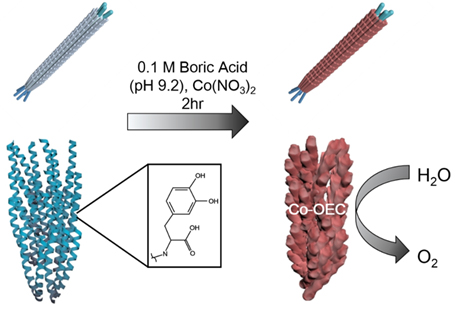 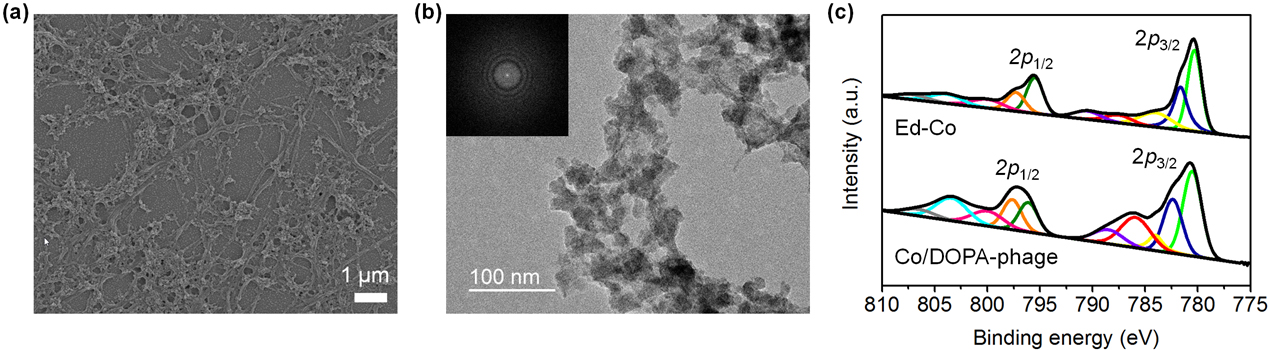 Mass spectrometry on gold microshell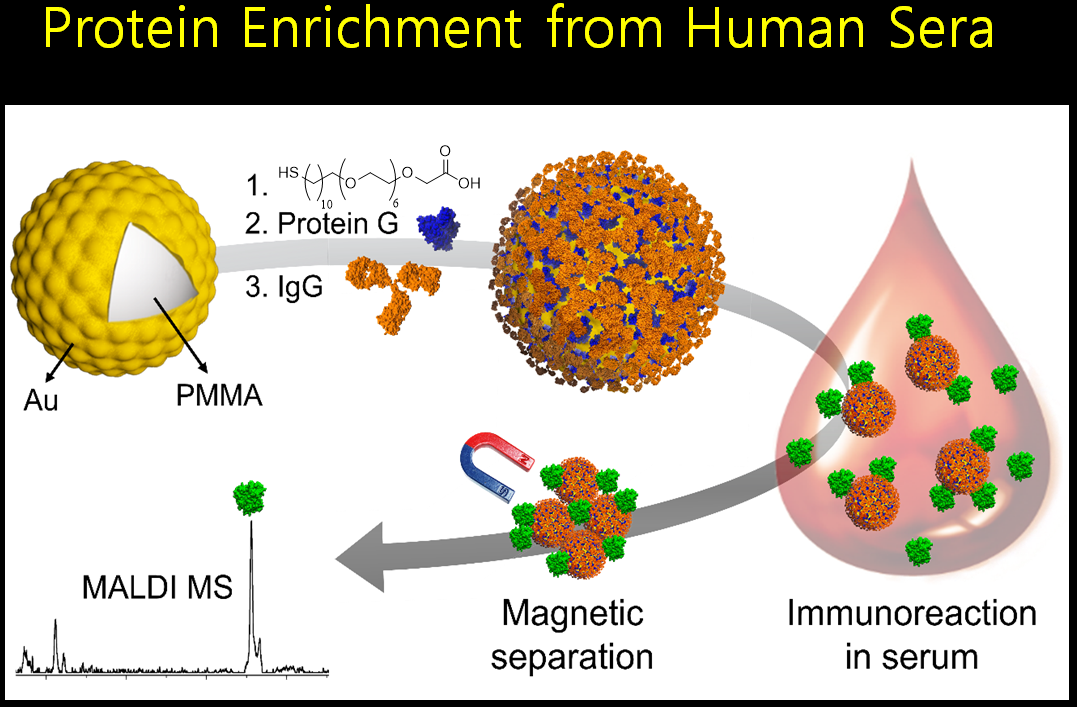 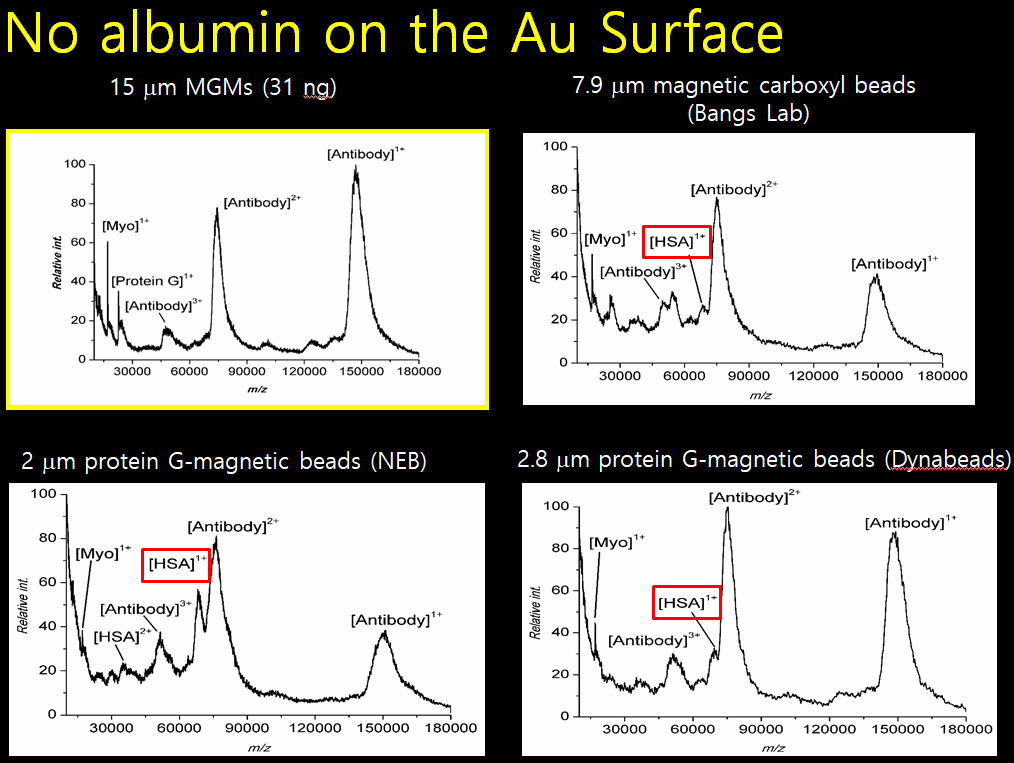 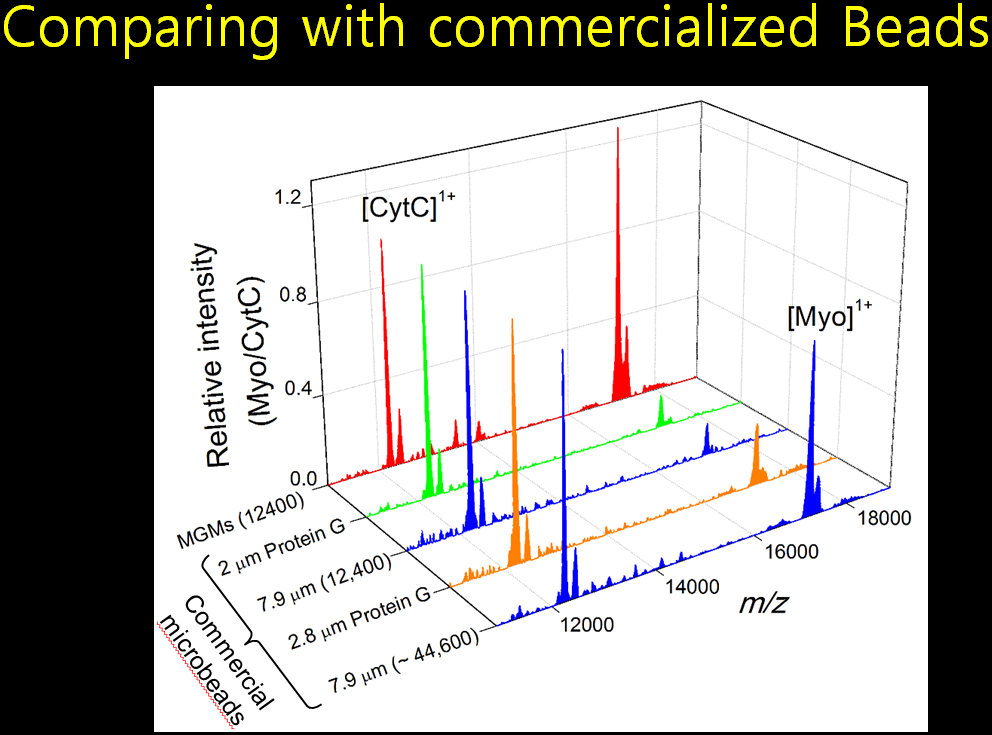 Photoelectrocatalysis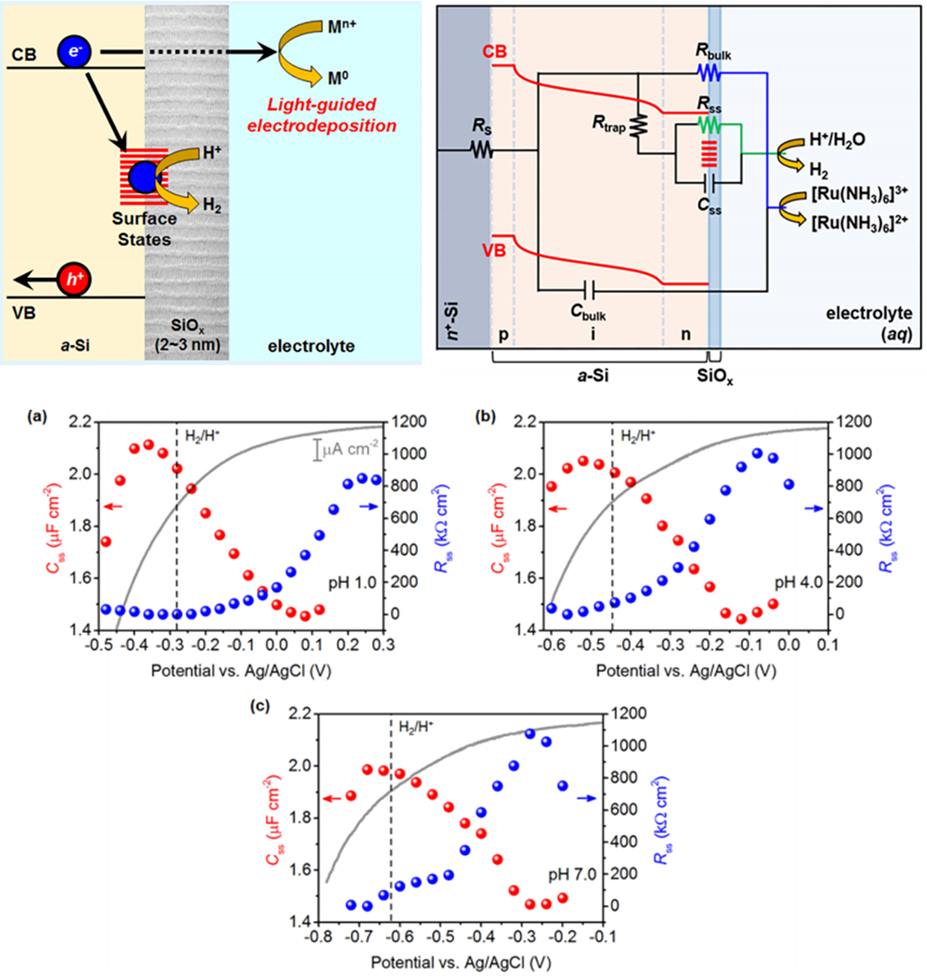
Semiconductor electrodes convert light energy into photovoltage, providing a sustainable way of valorizing various chemical substrates. From small-molecule activation chemistry such as oxygen evolution reaction (OER) and CO₂ reduction reaction (CO₂RR) to transformation of relatively complex organic molecules, photoelectrocatalysis is emerging as a central area of research in heterogeneous catalysis. Our group focuses on studying the novel role of semiconductor electrodes in photoelectrocatalysis. In particular, we are exploring the effect of the characteristic charge-transfer mechanism of the photoelectrochemical system on reaction outcomes, such as product selectivity and reaction efficacy. SiO₂ Electrochemistry with an Insulator.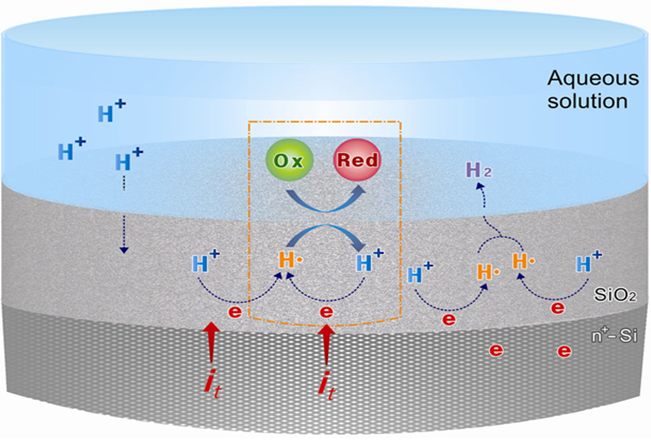
How can nature and living organisms induce chemical reactions, which can be considered as somewhat violent and destructive for living organisms, within their minute, delicate, and fragile internal environments? How can we artificially implement or explore “the magical reactions of nature” that occur with only small perturbations inside their microvolumes, such as currents, voltages, electromagnetic fields, and concentrations? Our research on silicon oxide (SiO₂) electrodes serves as a beacon for exploring such aspirations. SiO₂ is known as an insulator. But, when a negative potential is applied to the underlying conductive material, protons (H+) in the electrolyte infiltrate the fine gaps of the oxide and attempt to reduce. However, the narrow and fine gaps of the oxide prevent the reduced protons, i.e., hydrogen atoms (radicals, H·), from combining together to form hydrogen molecules (H₂). As a result, the SiO₂ electrode become a source of H·, unlike typical electrodes that predominantly generate H2 under reductive potential. These H·s sequentially initiate various organic/inorganic chemical reactions that are typically hard to occur in common electrochemical systems. Our main goal is to investigate what reactions occur and how they occur on such a SiO₂ electrode platform. Furthermore, by combining plasmonic nanomaterials with SiO₂ electrodes, we are expanding this platform to be controlled and regulated not only by electrochemical conditions but also by light. This provides additional constraints on the reaction medium at nanoscale localized sites, which resembles the reaction environment of nature and living organisms. To analyze what happens in these system, various electrochemical and other analytical techniques, such as voltammetry, electrochemical impedance spectroscopy, and product analysis, are conducted. Organic Electrochemistry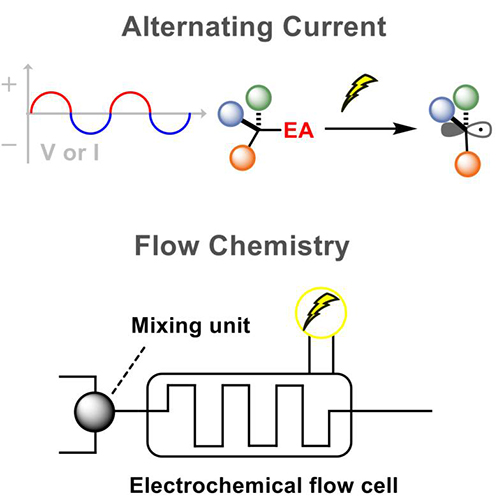
Organic electrochemistry is centered around controlling the oxidation and reduction processes of organic molecules at electrodes by applying electrical potential directly. This area of study utilizes the regulated movement of electrons to catalyze chemical reactions, offering a meticulous and effective approach to synthesizing and modifying organic compounds. - Alternating current in organic electrochemistry: The periodic reversal of charge flow in alternating current electrolysis (ACE) allows for versatile control over electrochemical reactions. This dynamic flow of charge enables precise manipulation of reaction conditions, leading to enhanced selectivity and efficiency in synthetic processes. - Flow cell system in organic electrochemistry: Integrating a continuous flow setup into electrochemical synthesis enables improved mass transfer, enhanced reaction rates, and better selectivity compared to traditional batch processes. It also promotes scalability, facilitating the transition from lab-scale to industrial production. * 클릭하면 연구 설명을 보실 수 있습니다. Microfluidics
Modern analytical chemistry is facing new challenges. One of the urgent targets to cope with the challenges is multiplex analysis requiring as small a sample volume as possible. In this situation, we paid attention to suspension arrays that employ microbeads. Bead-based suspension arrays for common use require enhanced sensitivity and effective prevention of non-specific adsorption, as well as miniaturization of the detection device. We have implemented virus-tethered gold microspheres for multiplex immunoassay applications, employing a DC impedance-based flow cytometer as a detection element. The advantages of virus-tethered gold microspheres, including excellent prevention of non-specific adsorption, are extended to signal enhancement arising from the large quantity of antibody loading on each virion, and to flexible movement of filamentous virus. Individual virus-tethered beads generate their own DC impedance and fluorescence signals, which are simultaneously detected by a chip-based microfluidic flow cytometer. This system successfully realized multiplex immunoassays involving four biomarkers: cardiac troponin I (cTnI), prostate specific antigen (PSA), creatine kinase MB (CK-MB), and myoglobin in undiluted human sera, elevating sensitivity by up to 5.7-fold compared to the beads without virus. Constructive integration between filamentous virus-tethered Au-layered microspheres and use of a microfluidic cytometer suggests a promising strategy for competitive multiplex immunoassay development based on suspension arrays. RED  
의견세 가지 분류 중에서는 Analytical platform에 속한다고 생각합니다. 과거에 RED를 bipolar electrode 센서의 power source로 결합하는 연구를 진행했었고, ionic 소자의 ionic power로 활용가능하다는 연구를 통해 미래의 생체분석장치와의 결합가능성도 보였습니다. 현재는 RED 자체를 분석장치로 활용하는 연구도 하고 있기 때문에 analytical platform 개발에 속한다고 생각합니다. Reverse electrodialysis (RED) is a energy generating system from the salinity gradient of sea water and river water. We focus on the fact that RED is an ideal power source surrogate to couple with various ionic devices without the need of any metal electrodes. Thus, we developed a miniaturized RED to be used as a power source for a drug delivery system and that of sensors, Conceptual improvement was also made via a precipitation-based approach to enhance its practicality. Now, we are attempting to expand its applications to a wide range of ion-based platforms. Analytical platform3D Interdigitated array Electrodes(3D IDA)Sensitivity is significant criteria for biosensors. We devised three-dimensional interdigitated array (3D IDA) platform for amplifying the current signal to enhance the sensitivity of chip-based electrochemical immunosensors. The 3D IDA consists of one pair of IDA which are positioned on the bottom and the ceiling facing each other in a microfluidic channel. Comparing with the case of feedback mode and non-feedback mode, the current signal of feedback mode was ca. 100 times higher than that of non-feedback mode. We also demonstrated the 3D IDA chip that operate as electrochemical immunosensors without reference and counter electrodes, which are essential components to control appllied potential but prevent users from being free to detect targets on-site. Electron transfer mediator is immobilized on the one pair of working electrodes and the electrochemical potential of the electrodes are defined by the formal potential of the mediators in 2-electrode (2E) system. Therefore, the 3D IDA in 2E system constitutes a simple platform for sensitive electrochemical detection and sheds light on miniaturized multiplex immunoassay field.  Iontronics
Iontronics, as the name reminds us of its meaning, mainly deals with electrical signal processing based on ions as signal carriers under aqueous, physiological conditions, and thereby aims to realize a bio-inspired information processing units. For the purpose of a sophisticated control of ions, positively- or negatively charged polyelectrolyte gels are used as a key component in the microfluidic chip. By optimizing the electrochemical circuit design and materials, we demonstrated that systematic combinations of such hydrogels produced the first aqueous microfluidic ion diode, digital logic gates and a polyelectrolytic junction field effect transistor (pJFET). Recently, we have constructed a full ionic circuitry with combination of reverse electrodialysis (RED) as an ionic power source, which is completely composed of non-electronic materials. We envision futuristic ionic devices capable of neurological signal processing functions on biocompatible substrates such as starch paper. Virtual ElectrodeVirtual Electrode
When the semiconductor absorbs photons of high enough energy, electron−hole pairs are created. Among them, the minority carriers migrate to the semiconductor surface and react with the redox species in the solution. Most photoelectrochemistry research has been focused on the production of environmentally friendly fuels taking advantage of the power of semiconductors, to convert solar energy into chemical energy. However, the characteristics of the semiconductor we are interested in is the 'transient' nature of photo-generated excitons. Excitons exclusively exist when and where the light illuminates. Therefore, the photoelectrochemical reactions are also spatiotemporally confined to the illumination. Thus, it is possible to induce a “virtual” electrode on the semiconductor substrate wherever and whenever we want without complicated electrical wiring process or the electrode-positioning procedure. So far, we have showed some applications based on virtual electrode including large scale, maskless metal patterning with amorphous silicon and microscale biomolecule detection with hematite photoanode.
Bio Fuel Cell
Enzymatic biofuel cell is fuel cell that utilizes enzymes as biocatalyst to oxidize its fuel such as glucose and reduce its oxidant to water to harvest energy. At anode, the electrons are separated from the fuel molecules by enzyme and transfer to the anode electrode. At cathode, those electrons transfer to the enzyme and react with the oxidant.
Anode와 cathode를 각각 dialysis membrane으로 package한다. dialysis membrane의 pore size는 glucose와 oxygen이 membrane을 통과하여 자유롭게 이동하기에 충분하다. 큰 사이즈의 단백질이 전극에 직접적으로 노출되는 환경을 제한함으로써 package는 전극 표면이 오염되지 않도록 한다. Packaged BFC를 동물 체내에 이식하면 체내에 존재하는 glucose와 oxygen을 연료로 에너지를 생성할 수 있다. Multi-Phase Electrochemistry
The Br-/Br2 redox couple in aqueous solution are being studied for redox flow batterys (figure 1). N-methyl-N-ethyl pyrrolidinium bromide (MEPBr) is used in Bromide redox system as bromine-complexing agent, forms an immiscible emulsion to overcome the self-discharge, corrosion and toxicity of Bromine. In recent studies, It is revealed that Bromide is oxidized in not only aqueous solution but also MEPBr2n+1, and there is a large amount of Bromide in the MEPBr2n+1 phase. In addition, We observed that when a single droplet of MEPBr2n+1 collides with electrode, bromide oxidizes immediately and spike-shaped current is occurred using in situ optical and electrochemical tools (figure 2). In the future, we will going to study electrochemistry of single droplet entity and compare the difference from the emulsion which is ensemble of the droplets. Synaptic Interface
Neurons communicate with each other using chemical signals through synapses. We envision a future where communicating with neurons is much easier. With this hope, we propose Janus synapses as a novel neural interfacing method. Just as neurons do, electronic devices and single neurons can establish connections for chemicalcommunication through synapses. This method involves pairing single neurons with counterpart electrodes. These electrodes induce the neurons to form synapses, differentiating intoeither presynaptic or postsynaptic terminals. 
One strategy we adopt is establishing synaptic connections between neurons and electrodes mediated by synaptic cell adhesion. Synaptic cell adhesion molecules are known to be involved in synapse formation. We have genetically engineered several of these molecules, including neuroligin (NLG), slitrk, and neurexin (NRX), to bind to electrode surfaces. Over the past several years, our lab has evaluated the synaptogenic abilities of these recombinant proteins. Interestingly, the recombinant postsynaptic proteins (neuroligin1, neuroligin2, and slitrk3) seems to induce different types of presynaptic terminals even when immobilized on artificial substrates such as electrodes and microspheres. These synaptic types include glutamatergic (excitatory), GABAergic (inhibitory), and dopaminergic. In this system, a presynapse forms spontaneously on a functionalized electrode, and exocytotic chemicals are directly released to the electrode. This allows chemical signals to be converted into electrical signals. With synapse type specificity, we monitor neuronal signals, particularly neurotransmitters, in Janus synapses using electrochemical methods. The Janus synapse is expected to be very robust against neuronal migration and glial penetration because the induced synapse is firmly bound to the electrode surface through protein-protein interaction. 
Iontronic (ionic) or electrical stimulation methods are commonly used in neuroscience research for studying neuronal activity and modulating neural function. Recently, our laboratory has discovered an innovative application of inverted ion current rectification phenomenon in stimulating neurons through devices, presenting promising advantages. This approach enables quantitative neurotransmitter delivery with high temporal resolution, negligible leakage, and no mechanical stimulation by using a polyelectrolyte-filled micropipette. Electrontransfer kinetics
Investigating the electron-transfer kinetics of rapid outer-sphere reactions using voltammetric techniques presents a significant experimental hurdle. This is primarily due to the fact that voltammetric measurements only detect heterogeneous kinetics effectively when the rates of electron transfer and mass transport to the working electrode reach a similar level, a condition that is hard to meet for fast reactions with standard electrochemical equipment. We have addressed this challenge by fabricating two parallel electrodes facing each other with narrow gaps down to 10 nm, allowing us to observe the redox behavior of electrochemically active molecules confined between them. This platform enables precise verification and control of the electrode geometry, ensures stable operation, and provides opportunities for analyzing electron transfer kinetics within an extended potential window. FTEIS
Fourier transform electrochemical impedance spectroscopy (FT-EIS) can be utilized as a powerful tool to a number of applications where the change of components in equivalent circuits should be examined in real-time manner. This technique allows for a very short EIS measurement time by applying a small (5~15 mV) voltage step signal to the system, and thereby calculating impedance values for the frequency range of interest through mathematical signal processing. Among its prospective applications are the fundamental study of electrochemical system, transient electrode process such as etching, electroplating, corrosion, and usages as a novel analytical platform. Probe Electrochemistry 
We have also installed sophisticated Scanning Ion Conductance Microscopy (SICM) and Scanning Electrochemical Cell Microscopy (SECCM) instruments based on LabVIEW, which allow us to conduct electrochemical experiments in various ways different from conventional SICM/SECCM. Recently, we have introduced polyelectrolyte gel-filled pipettes to SICM/SECCM, analyzing electrochemical interfaces such as measuring surface charge density or local potential of zero charge. Phage ElectrochemistryApplication of Filamentous Bacteriophage& Gold microshell
       Filamentous bacteriophage as a template for OER catalyst    Mass spectrometry on gold microshell   Photoelectrocatalysis
Semiconductor electrodes convert light energy into photovoltage, providing a sustainable way of valorizing various chemical substrates. From small-molecule activation chemistry such as oxygen evolution reaction (OER) and CO₂ reduction reaction (CO₂RR) to transformation of relatively complex organic molecules, photoelectrocatalysis is emerging as a central area of research in heterogeneous catalysis. Our group focuses on studying the novel role of semiconductor electrodes in photoelectrocatalysis. In particular, we are exploring the effect of the characteristic charge-transfer mechanism of the photoelectrochemical system on reaction outcomes, such as product selectivity and reaction efficacy. SiO₂ Electrochemistry with an Insulator.
How can nature and living organisms induce chemical reactions, which can be considered as somewhat violent and destructive for living organisms, within their minute, delicate, and fragile internal environments? How can we artificially implement or explore “the magical reactions of nature” that occur with only small perturbations inside their microvolumes, such as currents, voltages, electromagnetic fields, and concentrations? Our research on silicon oxide (SiO₂) electrodes serves as a beacon for exploring such aspirations. SiO₂ is known as an insulator. But, when a negative potential is applied to the underlying conductive material, protons (H+) in the electrolyte infiltrate the fine gaps of the oxide and attempt to reduce. However, the narrow and fine gaps of the oxide prevent the reduced protons, i.e., hydrogen atoms (radicals, H·), from combining together to form hydrogen molecules (H₂). As a result, the SiO₂ electrode become a source of H·, unlike typical electrodes that predominantly generate H2 under reductive potential. These H·s sequentially initiate various organic/inorganic chemical reactions that are typically hard to occur in common electrochemical systems. Our main goal is to investigate what reactions occur and how they occur on such a SiO₂ electrode platform. Furthermore, by combining plasmonic nanomaterials with SiO₂ electrodes, we are expanding this platform to be controlled and regulated not only by electrochemical conditions but also by light. This provides additional constraints on the reaction medium at nanoscale localized sites, which resembles the reaction environment of nature and living organisms. To analyze what happens in these system, various electrochemical and other analytical techniques, such as voltammetry, electrochemical impedance spectroscopy, and product analysis, are conducted. Organic Electrochemistry
Organic electrochemistry is centered around controlling the oxidation and reduction processes of organic molecules at electrodes by applying electrical potential directly. This area of study utilizes the regulated movement of electrons to catalyze chemical reactions, offering a meticulous and effective approach to synthesizing and modifying organic compounds. - Alternating current in organic electrochemistry: The periodic reversal of charge flow in alternating current electrolysis (ACE) allows for versatile control over electrochemical reactions. This dynamic flow of charge enables precise manipulation of reaction conditions, leading to enhanced selectivity and efficiency in synthetic processes. - Flow cell system in organic electrochemistry: Integrating a continuous flow setup into electrochemical synthesis enables improved mass transfer, enhanced reaction rates, and better selectivity compared to traditional batch processes. It also promotes scalability, facilitating the transition from lab-scale to industrial production. |